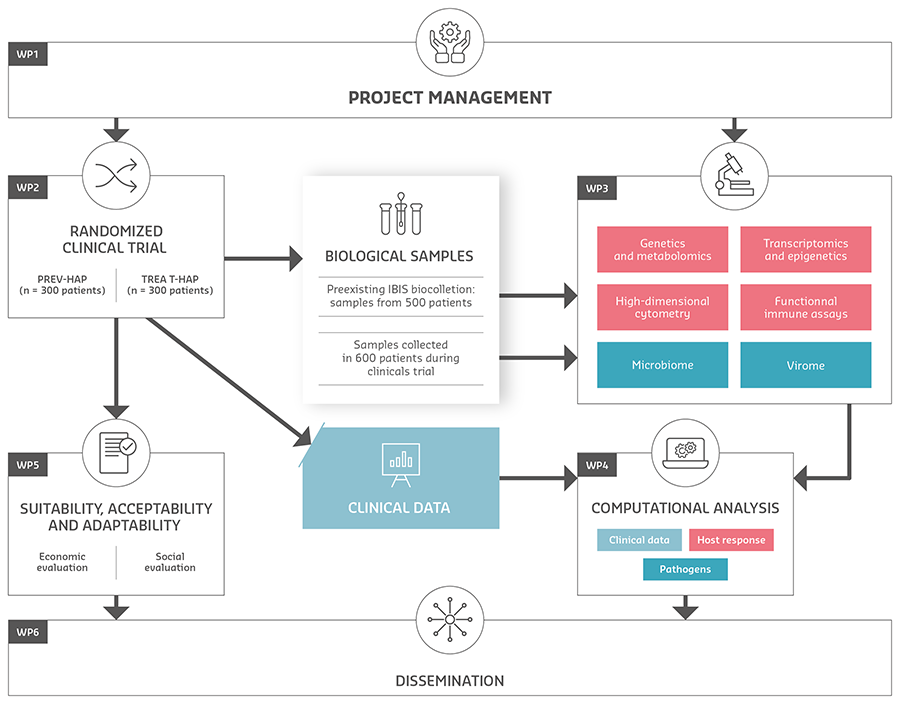
Within this 5-year project, two randomized clinical trials will be performed (WP2) to demonstrate the efficiency of rHuIL-12 or of IFN-γ for the prevention (trial 1 PREV-HAP), and for the treatment of HAP trial 2 TREAT-HAP). The respiratory and blood samples which are already available from existing cohorts and bio-collections, and those collected during these trials, will be analysed in WP3 by combining investigation of microbiome, virome and immunity to develop and validate biomarkers for patient stratification. These biomarkers will be combined in WP4 to build a clinico-biological score: the HAP2 toolbox, including clinical data (gender, age, etc.) and omics (including transcriptomics, genomics and epigenetics) for the prediction of HAP (high vs. low risk) and the response to host-targeted treatment (responders vs. non-responders). The suitability and acceptability of the proposed interventions for clinicians and patients as well as their cost-effectiveness will be measured during the clinical trials (WP5).
WP1 is dedicated to management and coordination of the project, while the dissemination, intellectual property management and exploitation of project results will be dealt with in WP6.