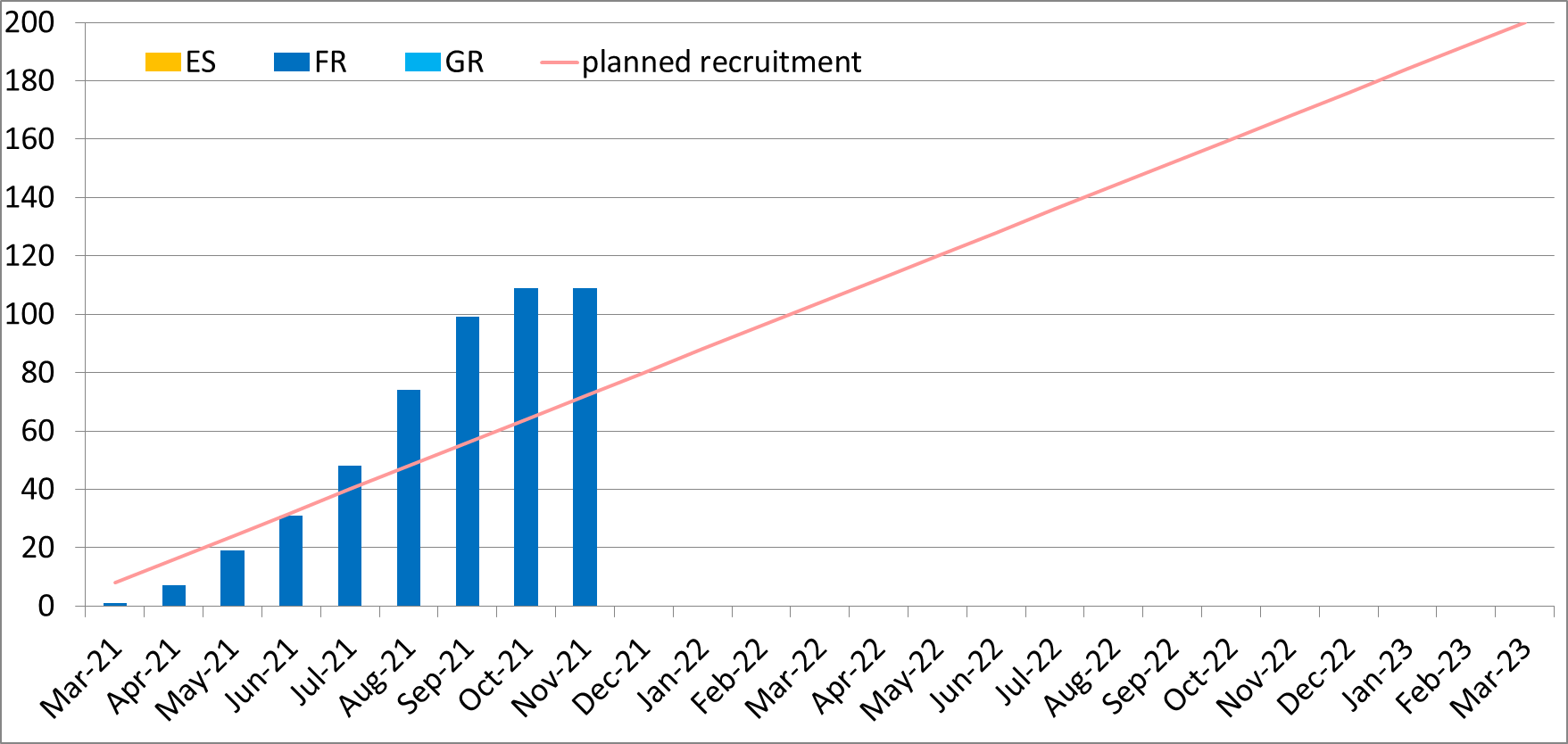
The PREV-HAP study
Full title:
Human Recombinant Interferon Gamma-1b for the Prevention of Hospital-acquired Pneumonia in Critically Ill Patients: a Double-blind, International, Phase 2, Randomized, Placebo-controlled Trial – the PREV-HAP Study
Summary:
This study is designed to establish whether human recombinant Interferon gamma 1b (rHuIFN-γ), as compared with placebo, can restore immunity in critically ill patients:
- reducing the rate of hospital-acquired pneumonia and
- improving outcomes in patients admitted to intensive care unit and requiring mechanical ventilation.
We also hypothesize that the in vivo investigations of the host-pathogens interactions can be used for the stratification of patients into high/low risk and responders/non-responders to host-targeted prevention of hospital-acquired infections.
The primary endpoint is the composite outcome of all-cause mortality at day 28 and/or the occurrence of hospital-acquired pneumonia within 28 days after randomization.